Data Analysis Task 3#
Learning Objectives#
In this lesson, we will:
“Use a library function to get a list of filenames that match a wildcard pattern.”
“Write a
forloop to process multiple files.”
Key points#
“Use
glob.glob(pattern)to create a list of files whose names match a pattern.”“Use
*in a pattern to match zero or more characters, and?to match any single character.”
Processing multiple files#
As a final piece to processing our inflammation data, we need a way to get a list of all the files
in our data directory whose names start with inflammation- and end with .csv.
The following library will help us to achieve this:
import glob
The glob library contains a function, also called glob, that finds files and directories whose names match a pattern. We provide those patterns as strings: the character * matches zero or more characters, while ? matches any one character. We can use this to get the names of all the CSV files in the current directory:
print(glob.glob('data/inflammation*.csv'))
['data/inflammation-05.csv', 'data/inflammation-11.csv', 'data/inflammation-10.csv', 'data/inflammation-04.csv', 'data/inflammation-12.csv', 'data/inflammation-06.csv', 'data/inflammation-07.csv', 'data/inflammation-03.csv', 'data/inflammation-02.csv', 'data/inflammation-01.csv', 'data/inflammation-09.csv', 'data/inflammation-08.csv']
As these examples show, glob.glob’s result is a list of file and directory paths in arbitrary order. This means we can loop over it to do something with each filename in turn. In our case, the “something” we want to do is generate a set of plots for each file in our inflammation dataset.
If we want to start by analyzing just the first three files in alphabetical order, we can use the sorted built-in function to generate a new sorted list from the glob.glob output:
import glob
import numpy as np
import matplotlib.pyplot as plt
filenames = sorted(glob.glob('data/inflammation*.csv'))
filenames = filenames[0:3]
for filename in filenames:
print(filename)
data = np.loadtxt(fname=filename, delimiter=',')
fig = plt.figure(figsize=(10.0, 3.0))
axes1 = fig.add_subplot(1, 3, 1)
axes2 = fig.add_subplot(1, 3, 2)
axes3 = fig.add_subplot(1, 3, 3)
axes1.set_ylabel('average')
axes1.plot(np.mean(data, axis=0))
axes2.set_ylabel('max')
axes2.plot(np.max(data, axis=0))
axes3.set_ylabel('min')
axes3.plot(np.min(data, axis=0))
fig.tight_layout()
plt.show()
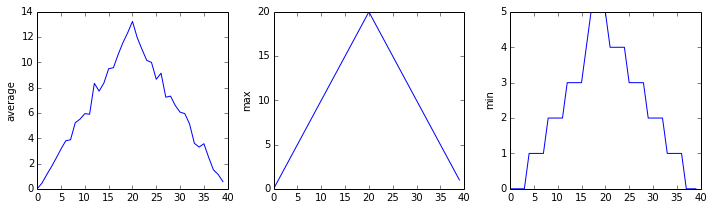
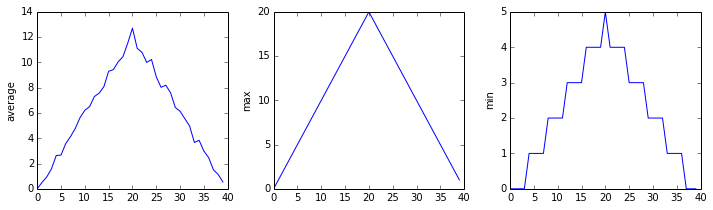
The plots generated for the second clinical trial file look very similar to the plots for the first file: their average plots show similar “noisy” rises and falls; their maxima plots show exactly the same linear rise and fall; and their minima plots show similar staircase structures.
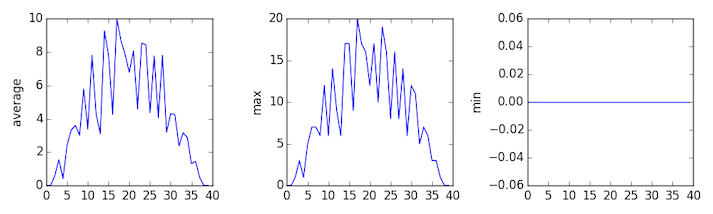
The third dataset shows much noisier average and maxima plots that are far less suspicious than the first two datasets, however the minima plot shows that the third dataset minima is consistently zero across every day of the trial. If we produce a heat map for the third data file we see the following:
We can see that there are zero values sporadically distributed across all patients and days of the clinical trial, suggesting that there were potential issues with data collection throughout the trial. In addition, we can see that the last patient in the study didn’t have any inflammation flare-ups at all throughout the trial, suggesting that they may not even suffer from arthritis!
Plotting Differences#
Plot the difference between the average inflammations reported in the first and second datasets (stored in inflammation-01.csv and inflammation-02.csv, correspondingly), i.e., the difference between the leftmost plots of the first two figures.
Solution
import glob
import numpy as np
import matplotlib.pyplot as plt
filenames = sorted(glob.glob('data/inflammation*.csv'))
data0 = np.loadtxt(fname=filenames[0], delimiter=',')
data1 = np.loadtxt(fname=filenames[1], delimiter=',')
fig = plt.figure(figsize=(10.0, 3.0))
plt.ylabel('Difference in average')
plt.plot(np.mean(data0, axis=0) - np.mean(data1, axis=0))
fig.tight_layout()
plt.show()
Generate Composite Statistics#
Use each of the files once to generate a dataset containing values averaged over all patients:
import glob
import numpy as np
# Get all filenames matching the pattern
filenames = glob.glob('data/inflammation*.csv')
# Initialize composite_data with the right shape
composite_data = np.zeros((60, 40))
# Loop over each file and add its data
for filename in filenames:
data = np.loadtxt(filename, delimiter=',')
composite_data += data
# Average over the number of files
composite_data = composite_data / len(filenames)
Then use pyplot to generate average, max, and min for all patients.
Solution
import glob
import numpy as np
import matplotlib.pyplot as plt
filenames = glob.glob('data/inflammation*.csv')
composite_data = np.zeros((60,40))
for filename in filenames:
data = np.loadtxt(fname = filename, delimiter=',')
composite_data = composite_data + data
composite_data = composite_data / len(filenames)
fig = plt.figure(figsize=(10.0, 3.0))
axes1 = fig.add_subplot(1, 3, 1)
axes2 = fig.add_subplot(1, 3, 2)
axes3 = fig.add_subplot(1, 3, 3)
axes1.set_ylabel('average')
axes1.plot(np.mean(composite_data, axis=0))
axes2.set_ylabel('max')
axes2.plot(np.max(composite_data, axis=0))
axes3.set_ylabel('min')
axes3.plot(np.min(composite_data, axis=0))
fig.tight_layout()
plt.show()
Conclusions#
After spending some time investigating the heat map and statistical plots, as well as doing the above exercises to plot differences between datasets and to generate composite patient statistics, we gain some insight into the twelve clinical trial datasets.
The datasets appear to fall into two categories:
seemingly “ideal” datasets that agree excellently with Dr. Maverick’s claims, but display suspicious maxima and minima (such as
inflammation-01.csvandinflammation-02.csv)“noisy” datasets that somewhat agree with Dr. Maverick’s claims, but show concerning data collection issues such as sporadic missing values and even an unsuitable candidate making it into the clinical trial.
In fact, it appears that all three of the “noisy” datasets (inflammation-03.csv,
inflammation-08.csv, and inflammation-11.csv) are identical down to the last value.
Armed with this information, we confront Dr. Maverick about the suspicious data and
duplicated files.
Dr. Maverick confesses that they fabricated the clinical data after they found out that the initial trial suffered from a number of issues, including unreliable data-recording and poor participant selection. They created fake data to prove their drug worked, and when we asked for more data they tried to generate more fake datasets, as well as throwing in the original poor-quality dataset a few times to try and make all the trials seem a bit more “realistic”.
Congratulations! We’ve investigated the inflammation data and proven that the datasets have been synthetically generated.
But it would be a shame to throw away the synthetic datasets that have taught us so much already, so we’ll forgive the imaginary Dr. Maverick and continue to use the data to learn how to program.

